√100以上 vinegar and baking soda balloon experiment observation 242255-Vinegar and baking soda balloon experiment conclusion
Results (what were the results of your experiment?) The bottle with the ½ cup of Diet Coke, when mixed with baking soda, inflated the balloon only 4 cm The bottle with the ½ cup of lemon juice, when mixed with baking soda, inflated the balloon 9 cm The bottle with vinegar, when mixed with baking soda, inflated the balloon 11 cmChemical reaction science experiments using baking soda and vinegar are a lot of fun and are great learning opportunities In this quick and easy experiment, we are going to use an endothermic chemical reaction and the resulting carbon dioxide caused by mixing baking soda and vinegar to inflate a balloon Materials Empty plastic or glass bottle Balloon 1 cupRead MoreLet the heavy end of the balloon dangle, so no baking soda goes in the bottle 4 Hold onto the balloon at the bottle neck, and pick up the heavy part of the balloon so that all the baking soda falls into the vinegar at the bottom of the bottle 5

Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download
Vinegar and baking soda balloon experiment conclusion
Vinegar and baking soda balloon experiment conclusion-Directions Using a funnel to add the baking soda to your balloon balloon Pour the vinegar into the bottle Carefully fit the balloon over the bottle opening, but do not drop the baking soda into the vinegar yet Once the balloon is completely over the cap, hold up the balloon and allow the baking soda to fall into the vinegar When the vinegar and baking soda combine there is a reaction between an acid and a base Vinegar is the acid and baking soda is the base This reaction between the two causes a gas called carbon dioxide to bubble and foam This gas having nowhere else to go, expands the balloon making the selfinflating balloon happen




Creating A Science Project Elmersscienceready Detroitmommies Com
//mocomicom/ presents Baking Soda, Vinegar and Inflating Balloon Blow Up Science Experiment for KidsREQUIREMENTS BalloonVinegarBaking SodaBottleThe kids thought that the baking soda and vinegar would make the biggest balloon because they've made baking soda paint bombs before, and know the power of this reaction!Baking soda is bicarbonate (NaHCO3) and vinegar is acetic acid (HCH3COO) One of the products this reaction creates is carbon dioxide, which makes the bubbles When the baking soda meets the vinegar, there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it
The science, behind this balloon baking soda experiment, is the chemical reaction between the base {baking soda} and the acid {vinegar} When the two ingredients combine the balloon experiment gets it's lift!Summary Vinegar is placed in a soda bottle and a baking soda filled balloon is attachedThe mixing of the baking soda and vinegar results in the balloon expanding Estimated Time 15 – minutes Materials Needed 1 5 L (169 oz) clean, empty, plastic soda bottle When you added the baking soda to the vinegar, the two combined to make carbondioxide gas, which inflated the balloon The expansion of the balloon changed the weight of your sealed flask because you and your entire experiment are submerged in a fluid air Just like water, air is a fluid, and fluids buoy up objects
The Baking Soda Balloon BlowUp Experiment Fill a water bottle onethird full of vinegar Put a funnel in the neck of a balloon, and hold onto the balloon neck and funnel Have your child pours in enough baking soda to fill the balloon halfway Slide the funnel out of the balloon and have your child hold the portion of the balloon with theAre you ready to learn about chemical reactions? Baking soda is a base and vinegar is an acid When these two materials mix, they create a chemical reaction that gives off CO2 gas This gas builds up inside the sealed bag, eventually becoming too great for the plastic baggie seal, which explodes open into the sky, releasing the gas



Science With Children Baking Soda And Vinegar Experiments




Baking Soda And Vinegar Lab Report Docx Baking Soda And Vinegar Lab Report Research Question What Is The Effect That Baking Soda Has On The Volume Of Course Hero
Baking soda Chemical name, sodium bicarbonate with formula NaHCO 3 Vinegar A dilute solution of acetic acid in water Acetic acid is also known as ethanoic acid, with the formula CH 3 COOH A beaker or jar The chemical reaction When baking soda is mixed with vinegar, something new is formed The mixture quickly foams up with carbon It's quite simple, combining vinegar and baking soda together is considered an acid base reaction Vinegar is acidic and baking soda is a base When you mix and acid with a base, two new chemicals form, carbonic acid and sodium acetate The new acid then decomposes into water and carbon dioxide which is like the bubbling you see in sodas Why did the balloon inflate?




Baking Soda And Vinegar Balloon Experiment



Www Northmasonschools Org Userfiles 97 Classes 2170 Bakingsodaandvinegarballoonexperiment Pdf Id 9642
That lift is the gas produced from the two ingredients is carbon dioxide or CO2 Directions Have your children scoop the baking soda into the balloon using the funnel Help your children put the vinegar into the flask using a pipette or small measuring cup Next, attach the balloon to the top of the flask;3 Stretch the open end of the balloon over the neck of the bottle Make sure it's on tight!



Www Mansfieldtexas Gov Documentcenter View 6005




Balloon Blow Up Science Experiment Children S Museum Of Sonoma County
In this experiment, students will combine vinegar and baking soda in a water bottle to create a chemical reaction that inflates a balloon!How to Play 1 Fill a clean, empty water bottle about onethird full with vinegar 2 Place a funnel in the neck of the balloon, and hold onto it as your child pours in enough baking soda to fill the balloon about halfway 3 Slip the funnel out of the neck of the balloon Ask your child to hold the portion of the balloon with the baking soda Adding vinegar to the Baking soda creates a chemical reaction The Baking soda is a base, while the vinegar is an acid When the two combine they create carbon dioxide (CO2) The gas rises up and tries to escape through the soft drink bottle Since the exit is sealed with balloon, the gas escapes into the balloon and blows it up




Is This A Chemical Or Physical Reaction




Baking Soda Vinegar Balloon Worksheets Teaching Resources Tpt
1 teaspoon baking soda 3 tablespoons of vinegar Clean a 1 liter bottle and let dry Using a funnel, add 1 teaspoon of baking soda to the bottle Place the small end of the funnel into the opening of the balloon Hold carefully and pour the vinegar into the balloonMake sure not to pour the baking soda into the vinegar!When you mix baking soda (sodium bicarbonate, NAHCO 3) and vinegar (Acetic Acid, CH 3 COOH) carbonic acid is quickly created This is what caused the bubbles you saw Carbonic acid (H 2 CO 3) is very unstable and breaks down into water and carbon dioxideCarbon dioxide is a gas and needs lots of room, so it rushes into the balloon




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Wait Weight Don T Tell Me Chemistry Earth Science Science Activity Exploratorium Teacher Institute Project
Conclusion The experiment and result of it supported our hypothesis that the bubbles would float on top of the mixture of the baking soda and vinegar It did this because when we combined the baking soda and vinegar it had a chemical reaction that produces carbon dioxide gas This layer of pure carbon dioxide was more dense than the bubbleEvidence When one fluid ounce of vinegar was used, it took 544 seconds for one tablespoon of baking soda to cease its reaction with the vinegar Towards the end of the reaction, I could see leftover baking soda that had not taken part in the reaction When two fluid ounces of vinegar was used, the length of the reaction dropped to 465 secondsCreating a reaction between baking soda and vinegar is a classic science experiment that kids of all ages love to watch We've actually used the mixture in a few different science experiments ourselves including our green themed experiment and our soda bottle speed boats But adding a balloon to the mix just ups the fun factor and makes it seem like a brand new experiment all




How To Make A Heavy Balloon By Pakalana




Baking Soda And Vinegar Balloons
I use it as a review for exothermic and endothermic reactions, acids, and bases There's so many different scientific reasons that can be Carefully fit the balloon over the lip of the bottle so no baking soda is poured into the bottle yet When you are ready, hold the balloon up and let the baking soda pour into the vinegar3 Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet) 4 Once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar 5 Observe the chemical reaction and effect on the balloon 6 Record observations Bibliography




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Inflate A Balloon With Baking Soda And Vinegar Life Is Peachy Science Experiments For Preschoolers Preschool Science Science Experiments
In this experiment, we're going to learn how to blow up a balloon using baking soda and vinegar!👉 MORE htt Balloon STEM Activity Like our bottle rockets, in this activity we are capturing the CO2 gases that result from a baking soda and vinegar reaction Using our STEM skills we tested different ratios to see how it affects the inflating of our balloons We have done this experiment for Groundhog Day and Halloween Balloons Funnels Measuring spoon Start by putting the funnel into the balloon This will make it much easier to get the baking soda inside Add baking soda We added about 4 teaspoons to each balloon To get your balloons a bit bigger than ours, you could add 1 or 2 teaspoons more Next, use a funnel to pour some vinegar into your bottles



Baking Soda Vinegar Balloon Experiment Capturing Parenthood




Baking Soda And Vinegar Experiment Report
The baking soda/vinegar balloons is a fascinating demonstration of acid base chemistry Vinegar is water with about 3 percent of a chemical called Acetic acid Baking Soda is a compound called Sodium Bicarbonate, also known as Sodium Hydrogen Carbonate (NaHCO 3), and is a base So the reaction occursMeasure 15 ml of baking soda using a measuring spoon Pour the baking soda into the balloon using a funnel Measure 45 ml of vinegar and pour it into a water bottle Put the mouth of the balloon on the wine spout to keep the baking soda in the balloon1 Describe what happens when the vinegar was poured into the cup of baking soda Answers may vary, but students should mention release of a gas This is a typical chemical reaction in which an acid vinegar, reacts with a base baking soda, to produce a new chemical a salt 2 The gas produced in this reaction can put out fires




Baking Soda Vinegar Balloon Experiment Youtube



1
How to do the SelfInflating Balloon Experiment To keep this scientific, add the same amount of inflation material into each bottle The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equation baking soda ( sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide plus water plus sodium ion plus acetate ion The chemical equation for the overall reaction is with s = solid, l The science, behind this balloon baking soda experiment, is the chemical reaction between the base {baking soda} and the acid {vinegar} When the two ingredients mix together the balloon baking soda experiment gets it's lift!




Balloon Blow Up Science Experiment




Inflate A Balloon With Baking Soda And Vinegar Pbs Kids For Parents
Measure 15 tsp or 875 grams of baking soda Insert a funnel into the opening of a balloon and add the baking soda to the balloon through the funnel See image to the right Measure 10 tbsp or approximately 150ml of vinegar Add the vinegar to the empty bottle If you wish to add food coloring to the vinegar, add 35 drops The most basic observation would be that mixing baking soda and vinegar causes a chemical reaction the results in part of the solution becoming a gasUsing the funnel, put the baking soda into the balloon Pour the vinegar in the bottle Now attach the balloon to the opening of the balloon in such a way that it fits closely, without leaving any gap Hold the balloon up so that the baking soda falls into the vinegar inside the bottle




Experiment 4 Chemical Reactions What Happens In A Chegg Com




Science For Kids Chemical Reactions Using Baking Soda And Vinegar Buggy And Buddy
Pour the vinegar into the bottle Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet) Once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar Observe the chemical reaction and effect on the balloonThat lift is the gas produced called carbon dioxide or CO2 The experiment and result of it supported our hypothesis that the bubbles would float on top of the mixture of the baking soda and vinegar It did this because when we combined the baking soda and vinegar it had a chemical reaction that produces carbon dioxide gas What is the purpose of the baking soda and vinegar experiment?




Science The Scientific Method Lesson 3 The Scientific Method Balloon Experiment Ppt Download




Balloon Vinegar Baking Soda Worksheets Teaching Resources Tpt
The baking soda and vinegar reaction is actually two separate reactions The first reaction is the acid base reaction When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda




Balloon Baking Soda Vinegar Science Experiment For Kids




Baking Soda And Vinegar Balloons




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download




Baking Soda And Vinegar Balloons




Balloon Baking Soda Vinegar Kids Science




Expanding Balloon Room Ppt Download




Baking Soda Vinegar And Our Atmosphere Experiment Wltx Com




Balloon Baking Soda Vinegar Science Experiment For Kids



Theimag Org Wp Content Uploads 03 Baking Soda And Vinegar Balloon 1 Pdf




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download




Baking Soda And Vinegar Balloons




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me




Balloon Baking Soda Vinegar Kids Science




Creating A Science Project Elmersscienceready Detroitmommies Com




Balloon Vinegar Baking Soda Worksheets Teaching Resources Tpt




What S The Difference Between Baking Soda And Baking Powder American Chemical Society




Balloon Baking Soda And Vinegar Experiment Data Collection Sheet




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Science The Scientific Method Lesson 3 The Scientific Method Balloon Experiment Ppt Download




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Designing An Experiment Using Baking Soda And Vinegar Pdf Free Download




Chemical Or Physical Change Perkins Elearning




Balloon Baking Soda Vinegar Science Experiment For Kids




How To Inflate Balloon With Vinegar And Baking Soda Science4fun




Baking Soda And Vinegar Reaction Earth Day Science Experiment



1




Baking Soda And Vinegar Balloons




Baking Soda Vinegar Lab Worksheets Teaching Resources Tpt




Balloon Blow Up Science Experiment




Sharing Chemistry With The Community Limiting And Excess Reagents Chem13 News Magazine University Of Waterloo




Baking Soda And Vinegar Chemical Reaction Mister C Youtube




Balloon Baking Soda And Vinegar Experiment Data Collection Sheet




Baking Soda And Vinegar Balloon Experiment Science Projects For Kids Educational Videos By Mocomi Youtube




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Baking Soda And Vinegar Balloon Experiment Science Project Education Com




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Balloon Baking Soda Vinegar Science Experiment For Kids




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Balloon Blow Up Science Experiment




Designing An Experiment Using Baking Soda And Vinegar Pdf Free Download



3




How To Fill A Balloon With Baking Soda Vinegar 5 Steps With Pictures Instructables




Classroom Resources Inflating A Balloon With Chemistry ct




Use Vinegar And Baking Soda To Blow Up A Balloon Discovery Express




Science For Kids Chemical Reactions Using Baking Soda And Vinegar Buggy And Buddy




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download




Baking Soda Vinegar Lab Worksheets Teaching Resources Tpt
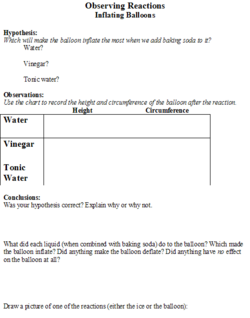



Observing Reactions Wikieducator




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me



1




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Science States Of Matter Jessica Clark Teacher Webpage




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities




Mrs Liebel S Classroom Balloon Blow Up Teaching Scientific Method Homeschool Science Experiments Science For Kids




Baking Soda Vinegar Experiment Worksheets Teaching Resources Tpt




Baking Soda And Vinegar Balloon Science Project Education Com




Balloon Baking Soda Vinegar Science Experiment For Kids




Science Club Reaction Of Vinegar With Bicarbonate Of Soda




Balloon Baking Soda Vinegar Kids Science




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Baking Soda Vinegar Balloon Worksheets Teaching Resources Tpt




Blow Up A Balloon Using Science Laughing Kids Learn




Baking Soda And Vinegar Balloon Experiment Youtube




Baking Soda And Vinegar Balloon Experiment




Baking Soda And Vinegar S Reaction Perkins Elearning




Fun And Easy Science In The Classroom Guest Blog Post The Applicious Teacher




Baking Soda Vinegar Balloon Experiment The Go To List




Designing An Experiment Using Baking Soda And Vinegar Pdf Free Download




Balloon Blow Up Science Experiment
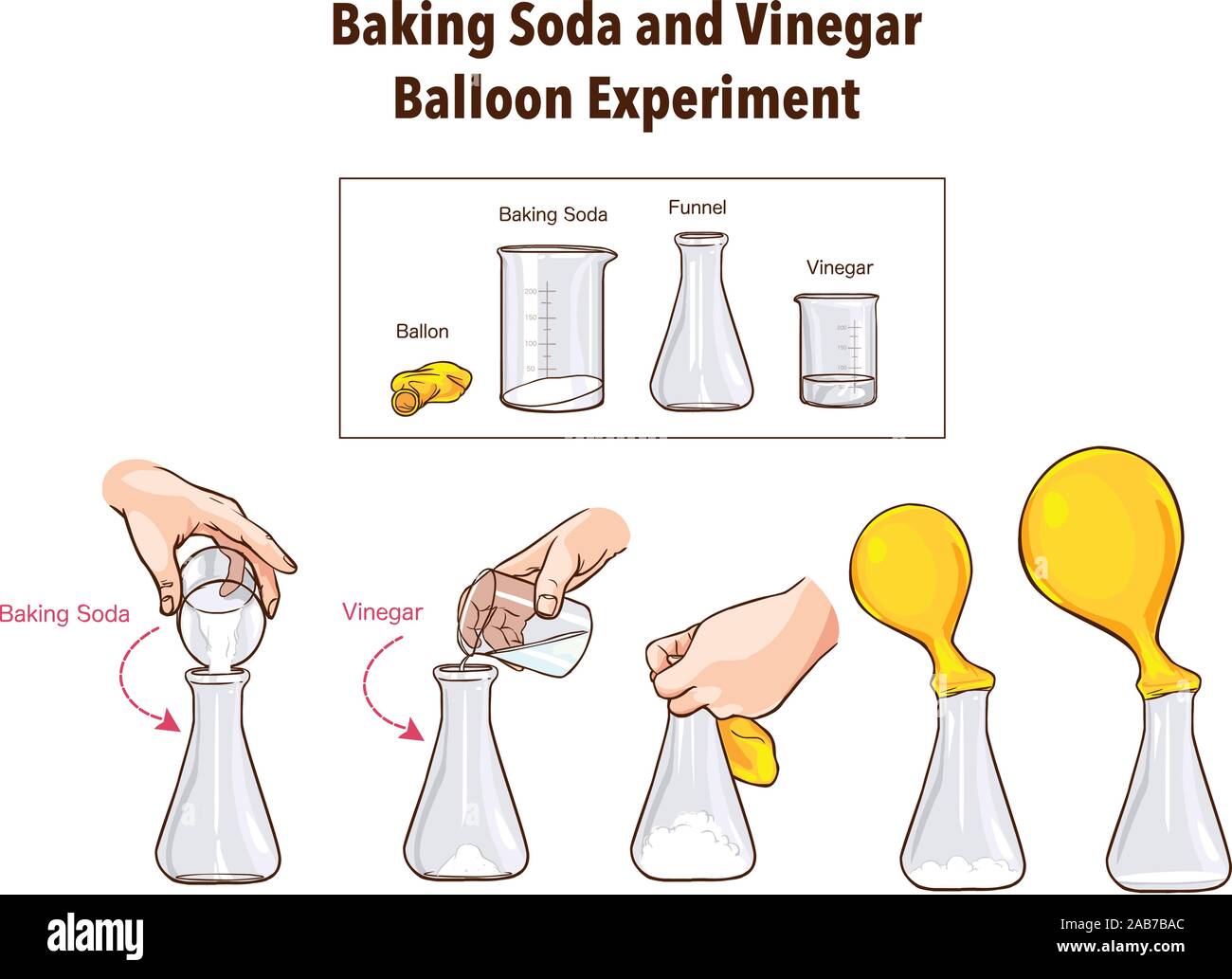



Baking Soda And Vinegar Balloon Experiment Science Stock Vector Image Art Alamy




Balloon Blow Up Science Experiment




Balloon Baking Soda Vinegar Science Experiment For Kids




Baking Soda And Vinegar Balloon Experiment For Kids Science Experiments Kids Kid Experiments Balloon Experiment
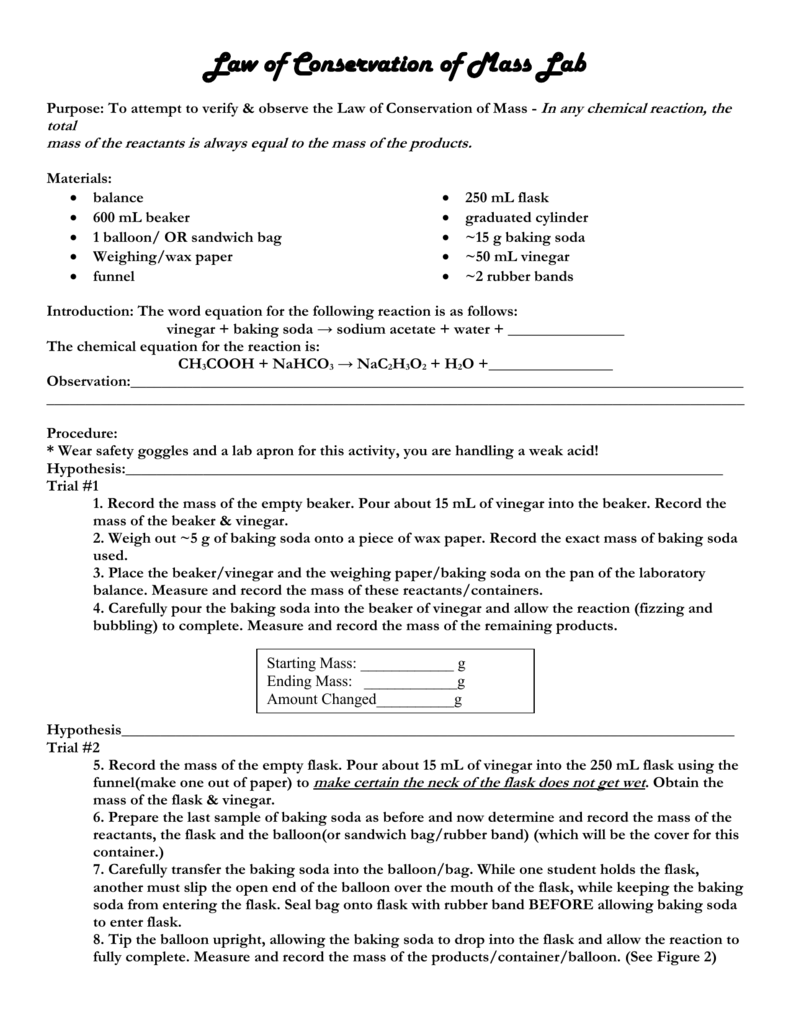



Law Of Conservation Of Mass Lab




Baking Soda Vinegar Balloon Worksheets Teaching Resources Tpt




Science Experiments For Kids Exploring Air Pressure Familyeducation




How To Inflate Balloon With Vinegar And Baking Soda Science4fun




Vinegar And Baking Soda Balloon Experiment Happy Brown House
コメント
コメントを投稿